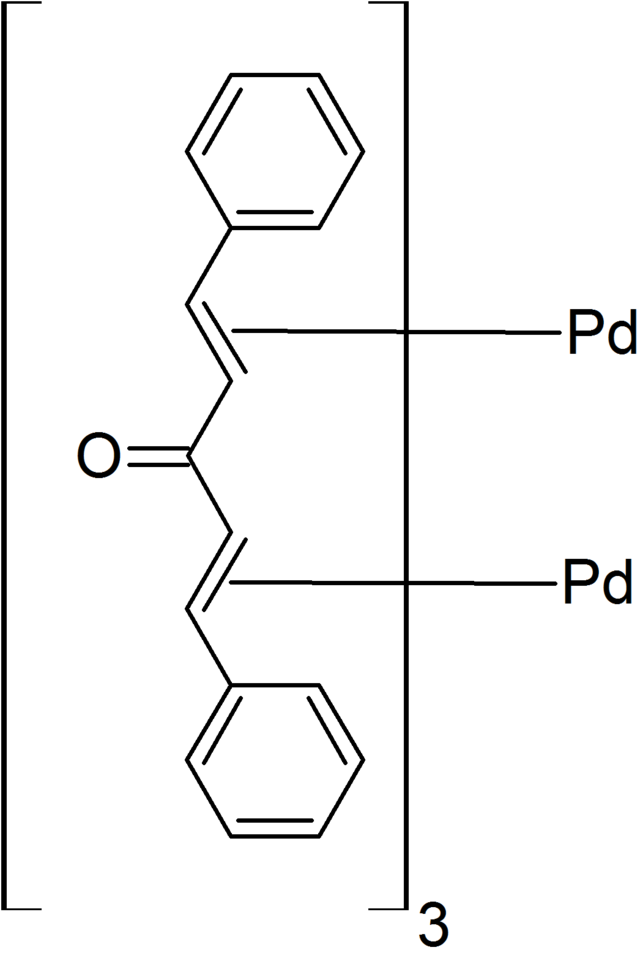A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles—the Case for Exploiting Pd Catalyst Speciation | Journal of the American Chemical Society

Pd(dba)2 vs Pd2(dba)3: An in-Depth Comparison of Catalytic Reactivity and Mechanism via Mixed-Ligand Promoted C–N and C–S Coupling Reactions | Organic Letters

Well-Defined Pdn Clusters for Cross-Coupling and Hydrogenation Catalysis: New Opportunities for Catalyst Design | ACS Catalysis

Exploiting Noninnocent (E,E)‐Dibenzylideneacetone (dba) Effects in Palladium(0)‐Mediated Cross‐Coupling Reactions: Modulation of the Electronic Properties of dba Affects Catalyst Activity and Stability in Ligand and Ligand‐Free Reaction Systems ...

Exploiting Noninnocent (E,E)‐Dibenzylideneacetone (dba) Effects in Palladium(0)‐Mediated Cross‐Coupling Reactions: Modulation of the Electronic Properties of dba Affects Catalyst Activity and Stability in Ligand and Ligand‐Free Reaction Systems ...

Exploiting Noninnocent (E,E)‐Dibenzylideneacetone (dba) Effects in Palladium(0)‐Mediated Cross‐Coupling Reactions: Modulation of the Electronic Properties of dba Affects Catalyst Activity and Stability in Ligand and Ligand‐Free Reaction Systems ...

Exploiting Noninnocent (E,E)‐Dibenzylideneacetone (dba) Effects in Palladium(0)‐Mediated Cross‐Coupling Reactions: Modulation of the Electronic Properties of dba Affects Catalyst Activity and Stability in Ligand and Ligand‐Free Reaction Systems ...

Pd(dba)2 vs Pd2(dba)3: An in-Depth Comparison of Catalytic Reactivity and Mechanism via Mixed-Ligand Promoted C–N and C–S Coupling Reactions | Organic Letters

Exploiting Noninnocent (E,E)‐Dibenzylideneacetone (dba) Effects in Palladium(0)‐Mediated Cross‐Coupling Reactions: Modulation of the Electronic Properties of dba Affects Catalyst Activity and Stability in Ligand and Ligand‐Free Reaction Systems ...

Pd(dba)2 vs Pd2(dba)3: An in-Depth Comparison of Catalytic Reactivity and Mechanism via Mixed-Ligand Promoted C–N and C–S Coupling Reactions | Organic Letters

Pd(dba)2 vs Pd2(dba)3: An in-Depth Comparison of Catalytic Reactivity and Mechanism via Mixed-Ligand Promoted C–N and C–S Coupling Reactions | Organic Letters